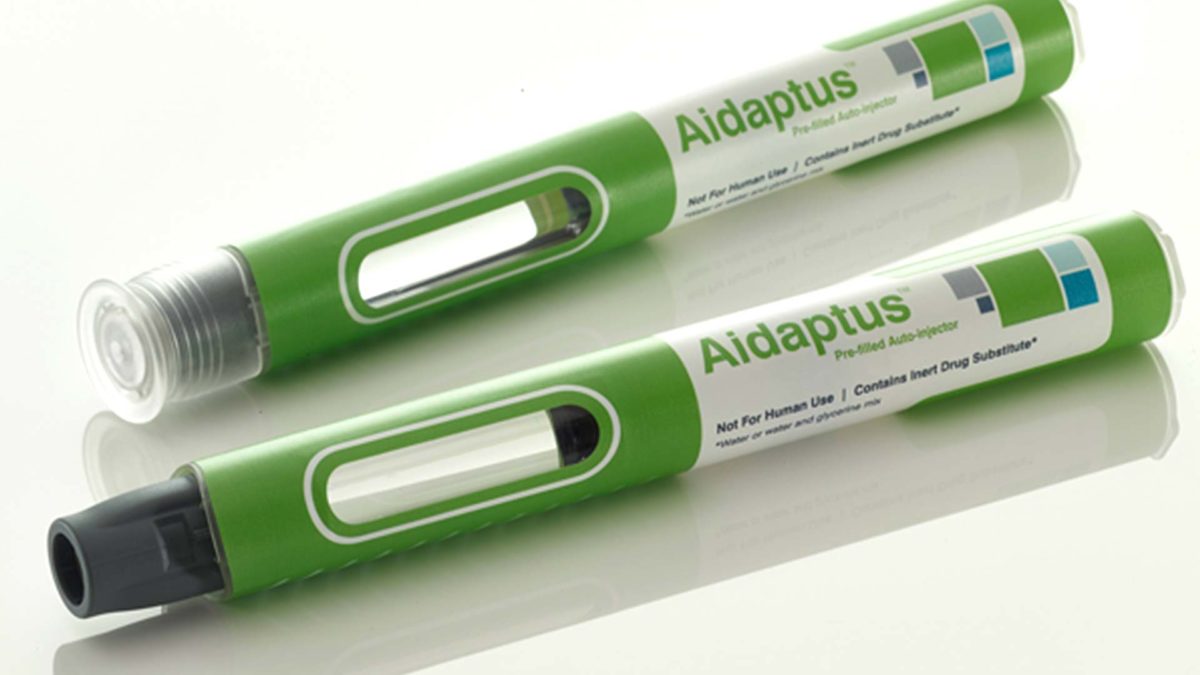
Owen Mumford launches new 2-step single-use auto-injector platform Aidaptus®
Industry first single-use auto-injector for both 1ml and 2.25 pre-filled syringes with versatile design for different fill volumes in the same device.
Owen Mumford Pharmaceutical Services, a division of Owen Mumford Ltd. has launched its new Aidaptus® auto-injector platform following successful completion of development. Aidaptus is a 2-step, spring-powered, single use auto-injector with a versatile design that accommodates both 1mL and 2.25 mL prefilled glass syringes in the same base device. It also features stopper sensing technology with a self-adjusting plunger that automatically adapts to the individual stopper positions and different fill volumes in each syringe, with no change parts required. The auto-injector is available with two different spring strengths to accommodate a variety of drug viscosities.
Aidaptus has an innovative, patient-centric design with automatic needle insertion that provides a simple and consistent user experience. The stopper sensing technology, coupled with the independent, two-phase needle insertion and drug delivery, significantly reduces any impact forces on the syringe, mitigating the risk of syringe breakages during use. With a needle that is shielded before, during and after use, Aidaptus provides reassurance to users who are new to auto-injectors as well as those who experience needle phobia. It can also give users confidence that the injection has been successfully completed with an audible notification at the start and end of the procedure. In addition, a bright yellow plunger rod is visible though a large window in the device confirming the end of injection.
Aidaptus is available in two base platform options: a clear outer body with colour overwrap permitting rapid selection of window size or traditional opaque housing, offering versatility for both branding and customisation as well as options for market segmentation and life cycle management. Its highly compact nature reflects the excellence in sustainable design and human factors engineering used throughout its development; coupled with consideration for ease of final assembly through application of design for manufacture (DfM) principles.
“Our Aidaptus auto-injector platform has been designed to facilitate the growing market for subcutaneous injections used to treat a variety of chronic diseases like rheumatoid arthritis, Crohns’ disease and multiple sclerosis,” said Michael Earl, Director, Owen Mumford Pharmaceutical Services. “With the increasing move towards treatment outside of the acute care setting, Aidaptus will help patients to self-administer their individual therapies using a simple and easy to use device. At the same time, this auto-injector can help to reduce complexity and risk in supply chain and final assembly for pharmaceutical and biotechnology companies’ combination products thanks to its ability to adapt to different syringe sizes and fill volumes. It is often the case that during development or life-cycle management of injectable drug products, changes in these parameters can occur. Now the device does not have to be changed as well.”
Aidaptus has been designed to meet the challenges of changes to drug formulation or injected volume and can therefore help reduce risk during drug development and lifecycle management.
For more information about the new OMPS Aidaptus® auto-injector platform, please visit: https://www.ompharmaservices.com/aidaptus/
More in Health

Helping Ordinary People do Extraordinary Things
B4 Members Hall Personal Training have recently renewed their B4 Membership. Executive Director, Becky Hall, explains more about Hall’s role in the lives of their clients and why renewing with B4 was a simple decision.

Owen Mumford: 70 Years of Innovation in Healthcare
Owen Mumford has been at the forefront of medical device innovation for over 70 years, pioneering solutions that enhance patient care and improve healthcare outcomes worldwide. Established in 1952 by Ivan Owen and John Mumford, the company has grown into a global leader in medical device design and manufacturing, with a commitment to quality, sustainability, and patient-centred innovation.

Activate Business School launches new education programme for aspiring health leaders
Activate Business School is delighted to announce it is launching a new award to help develop and train health and care staff across Buckinghamshire Oxfordshire and Berkshire (BOB).
From this author

Owen Mumford’s Near-Term and Net-Zero targets approved by the SBTi
Owen Mumford, a leader in the medical device industry and certified B Corp, has announced that the Science Based Targets initiative (SBTi) has approved its near-term science-based emissions reduction target. The medical device manufacturer has also committed to set long-term emissions reduction targets with the SBTi in line with reaching net-zero by 2045 – 5 years ahead of the UK’s net-zero target.

Owen Mumford acquires Empelvic
Owen Mumford Expands Pelvic Health Portfolio with Acquisition of Empelvic

Owen Mumford partners with Duopharma Biotech to distribute medical devices
Partnership with leading pharmaceutical company will supply diabetes and eye care devices in the Malaysia and Brunei markets

